Targeting Coenzyme A (CoA) genetic disorders
CoA Therapeutics is targeting Coenzyme-A for patients with PKAN, organic acidurias and other diseases of CoA sequestration
About CoA and its Biosynthetic Pathway
Coenzyme A (CoA) plays a crucial role in energy metabolism and is implicated in a large number of disorders, from ultra-rare diseases like pantothenate kinase-associated neurodegeneration, or PKAN, and organic acidurias (OAs), to common diseases such as Type 2 diabetes.
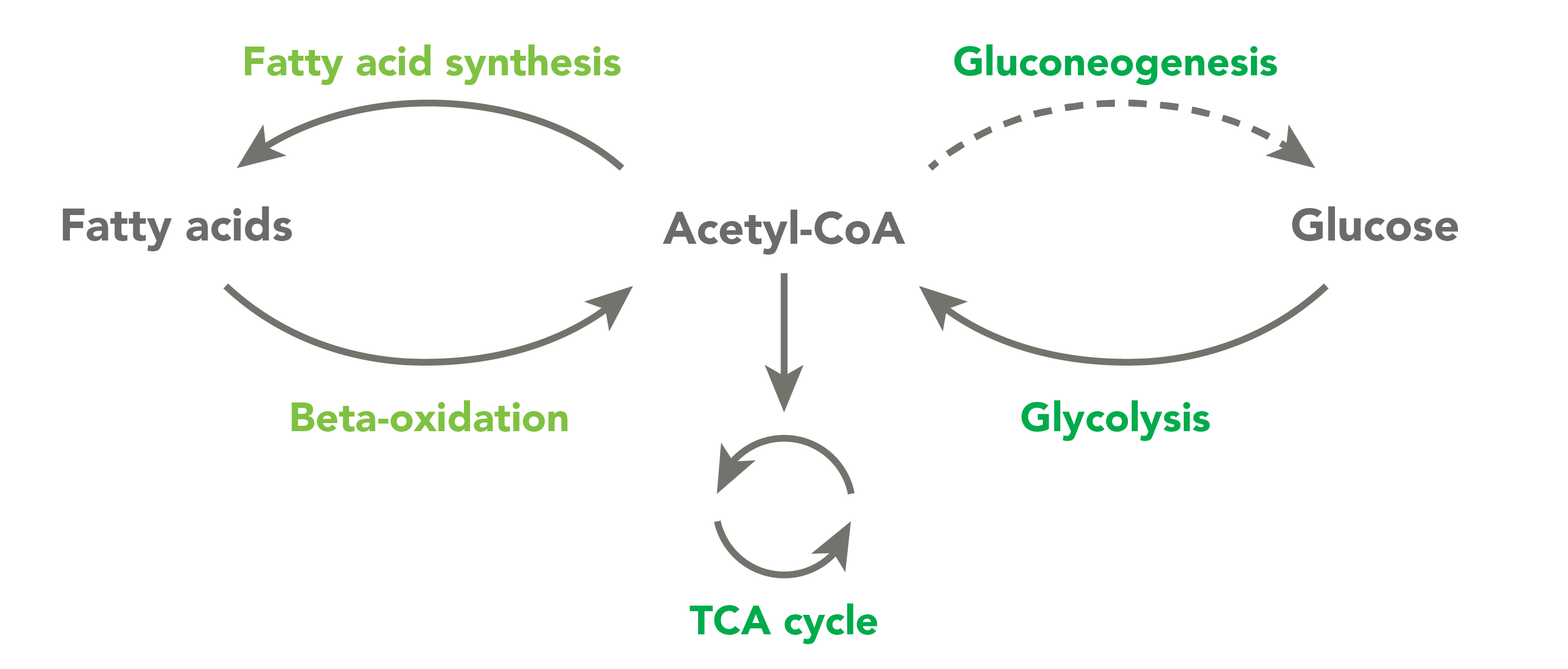
CoA and its related derivatives, which are called thioesters, are used by approximately 4% of cellular enzymes. CoA thioesters regulate the crossroads of energy metabolism. They are involved in some of the most important pathways responsible for breaking down metabolites and building key molecules necessary for healthy physiology. These pathways include the Krebs cycle, fatty acid synthesis and degradation, ketogenesis and steroidogenesis.
CoA Biosynthetic Pathway
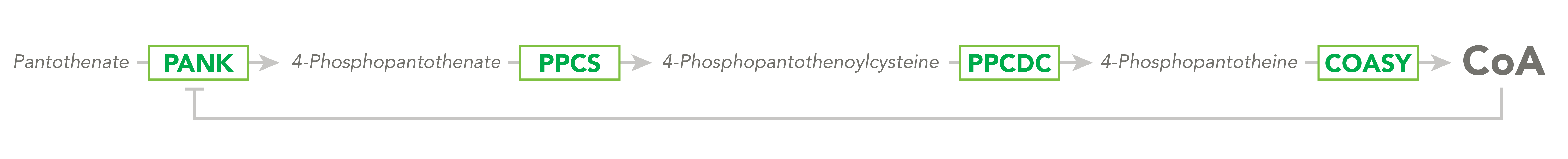
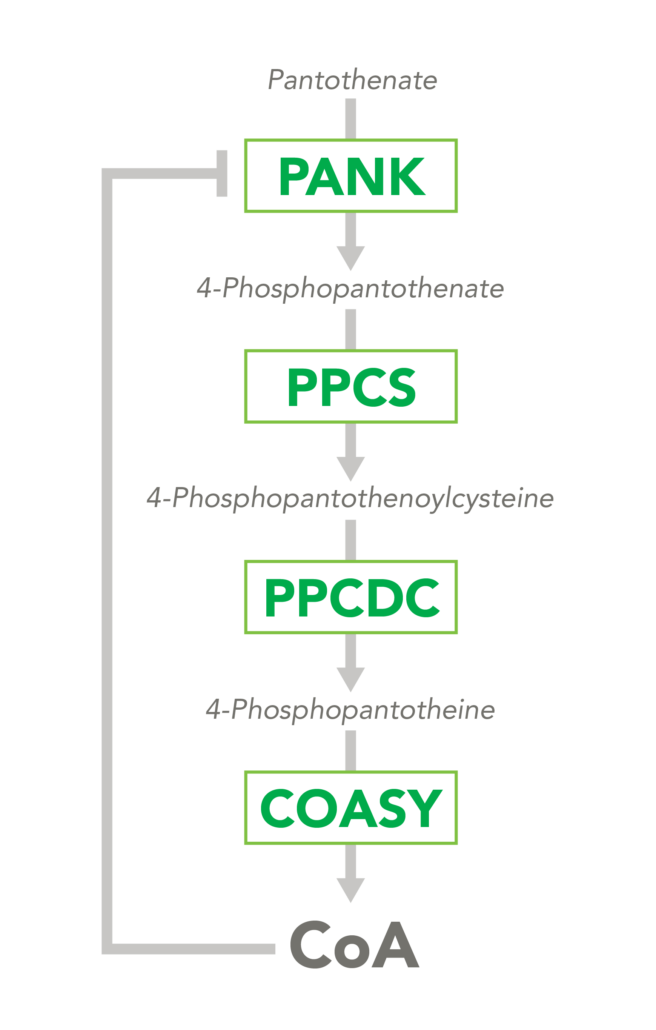
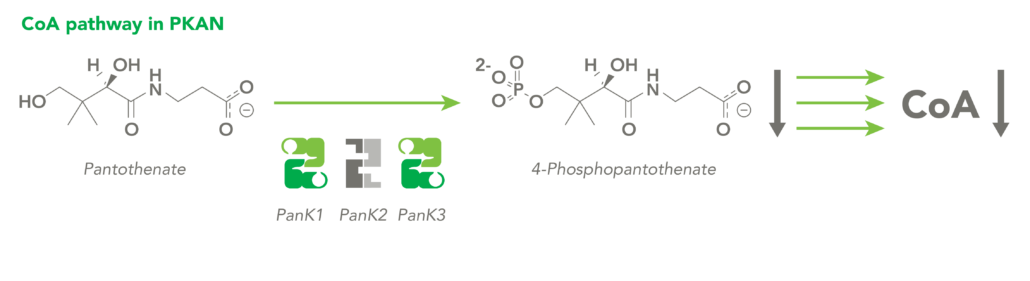
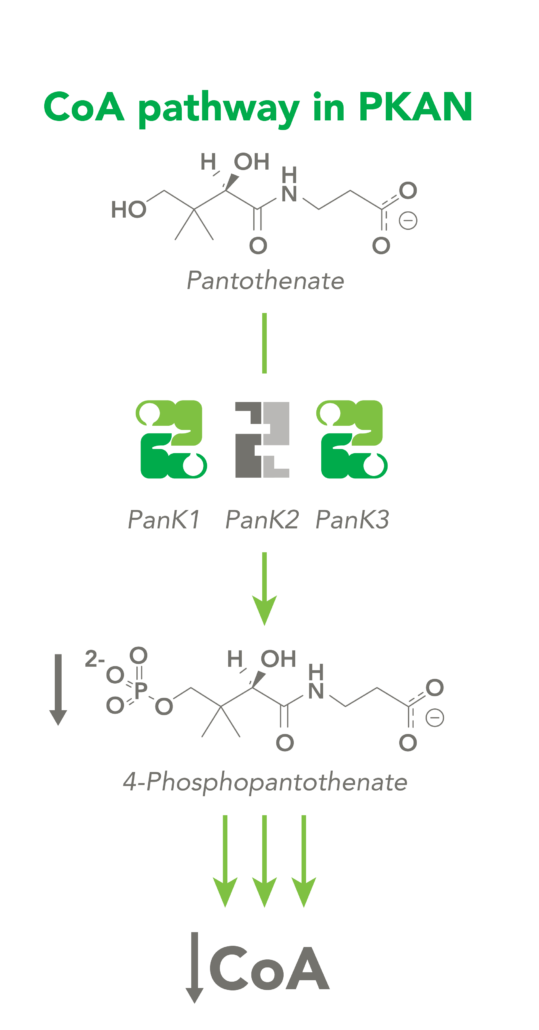
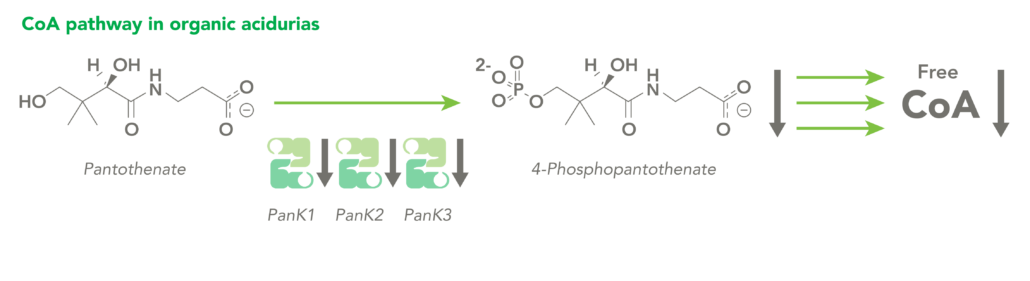
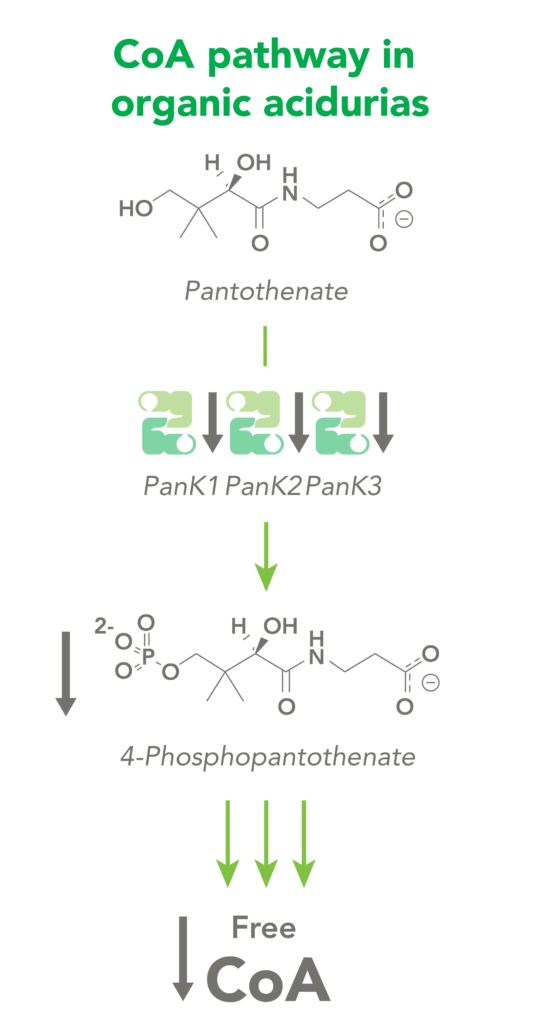
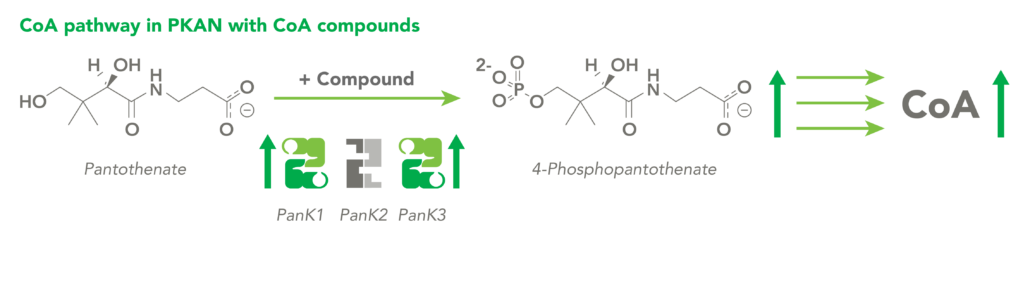
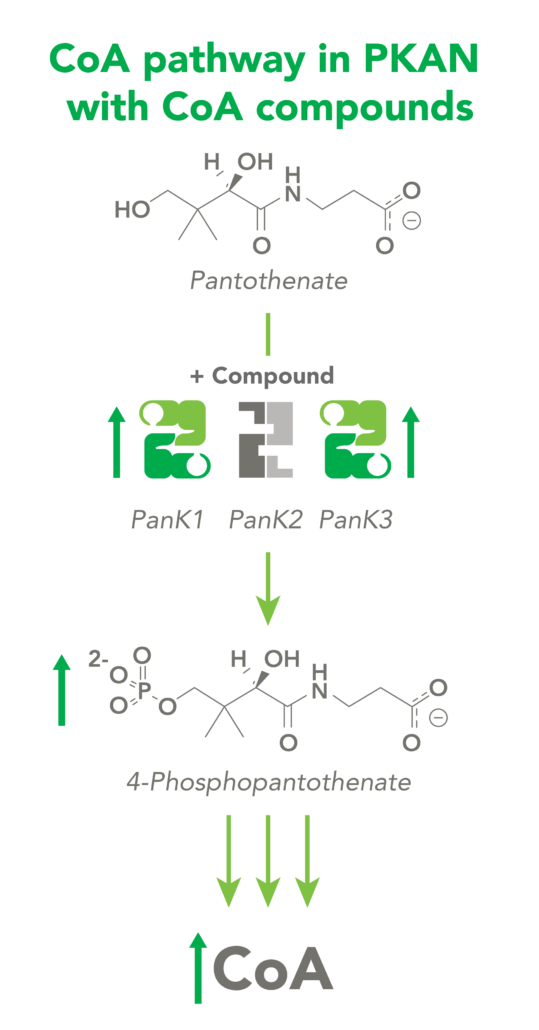
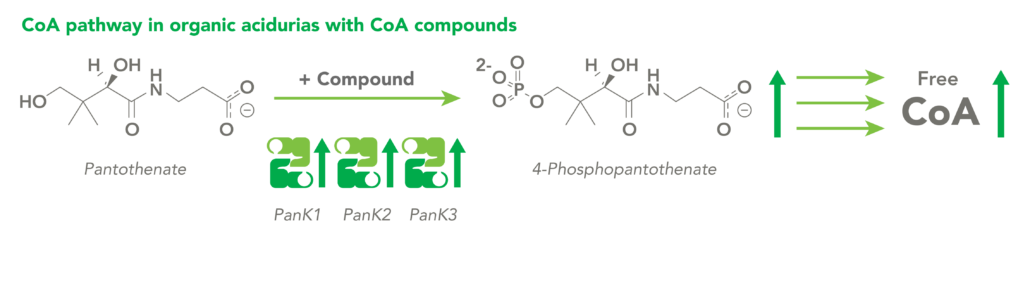
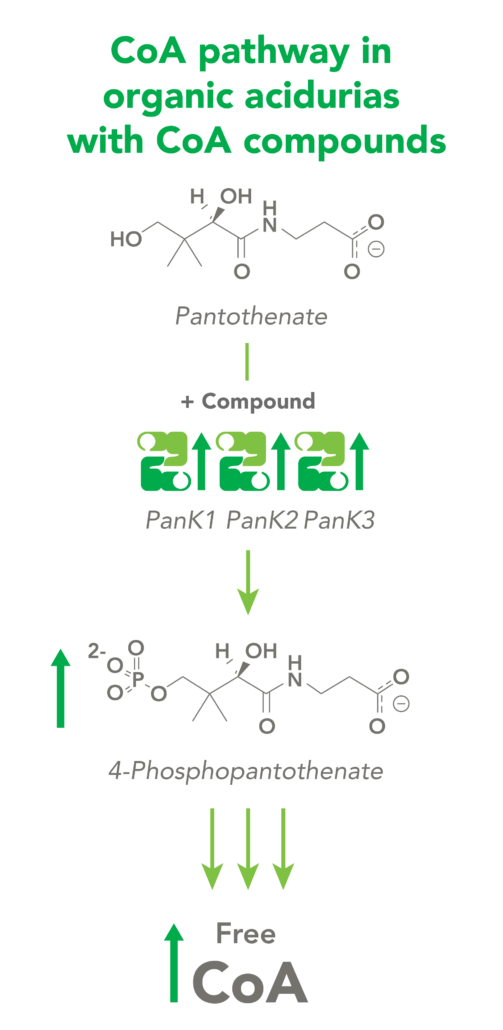
The CoA biosynthetic pathway begins with pantothenate (Vitamin B5), which is obtained through diet and the gastrointestinal microbiome. The sole biologic fate of pantothenate is CoA synthesis. Pantothenate is converted by pantothenate kinase (PanK) to 4’-phosphopantothenate, which is rapidly converted into CoA. CoA thioesters inhibit PanK function, providing a feedback mechanism for regulation of CoA levels.
About PKAN
Pantothenate kinase associated neurodegeneration, or PKAN, is the most common form of neurodegeneration with brain iron accumulation (NBIA). PKAN is a devastating disease that typically results in death in early adulthood. Individuals with PKAN typically develop symptoms before age 10 followed by a rapid deterioration in movement, speech and vision associated with neuronal degeneration. PKAN is estimated to affect up to 3 in every 1,000,000 people. There are currently no treatments approved for PKAN.
PKAN is an autosomal recessive genetic disorder caused by mutations in the pantothenate kinase 2 (PANK2) gene. The PanK2 enzyme plays a critical role in the synthesis of CoA in neurons; patients with PKAN either have a complete absence or a large deficiency of the enzyme activity, reducing neuronal CoA levels.
About organic acidurias
Organic acidurias (OAs), including propionic acidemia (PA) and methylmalonic acidemia (MMA), are autosomal recessive metabolic disorders in which normal amino acid metabolism is disrupted, causing a buildup of metabolic intermediates. OAs are associated with slow growth, lethargy, malnutrition, neurological damage and developmental delay. In different OAs, unique metabolic pathways are affected. It is estimated that organic acidurias affect five or six in every 100,000 births. The metabolic intermediates that accumulate in OAs can inhibit PanK enzymes, reduce cellular free CoA levels and thereby impact energy metabolism.
Our Targeted Approach
CoA Therapeutics is developing a novel small-molecule approach to modulate CoA levels by leveraging recent research about the Coenzyme A synthetic pathway. While PanK2 is inactivated in PKAN patients, other isoforms known as PanK1 and PanK3 are still active. This leads to decreased PanK activity and impaired CoA synthesis. Conversely, in organic acidurias, build-up of metabolic intermediates can inhibit the activity of all three isoforms of PanK and impair CoA synthesis. CoA Therapeutics’ small molecules can bind to all three PanK isoforms – although in PKAN, PanK2 is defective – prevent feedback inhibition, and thereby increase PanK activity and increase CoA synthesis.
CoA IS A MEMBER OF THE BRIDGEBIO FAMILY
BridgeBio is a team of experienced drug discoverers, developers and innovators working to create life-altering medicines that target well-characterized genetic diseases at their source. BridgeBio was founded in 2015 to identify and advance transformative medicines to treat patients who suffer from Mendelian diseases, which are diseases that arise from defects in a single gene, and cancers with clear genetic drivers. BridgeBio’s pipeline of over 20 development programs includes product candidates ranging from early discovery to late-stage development.